Web Content Viewer
Web Content Viewer
Treating Tumors: How The Game Has Changed
Published on 11/12/21
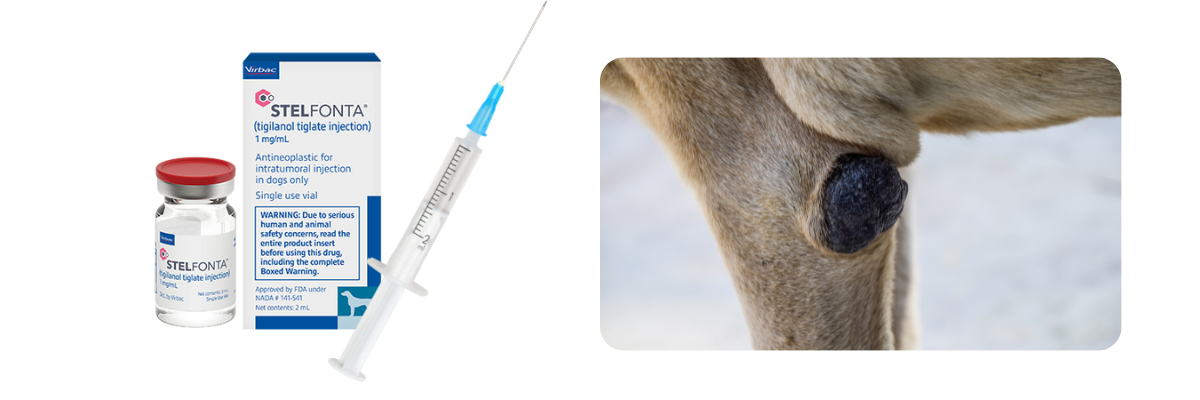
The game has changed for Mast Cell Tumor treatment.
The FDA approved STELFONTA® (tigilanol tiglate injection) as an intratumoral injection for treating canine mast cell tumors in November 2020.
Dr Chad Johannes, Veterinary Oncologist and Assistant Professor at Iowa State University described his experience with STELFONTA:
"In my 20-year career I've come across many products that strive to be innovative but delivering on that promise is challenging. STELFONTA brings a novel therapeutic mechanism and intratumoral delivery route to veterinary medicine. The efficacy and durability of response data in dogs with mast cell tumors are very promising. I think it will reshape how we approach local mast cell tumor control, especially in anatomical locations where obtaining adequate surgical margins are challenging."
STELFONTA is indicated for all grades of non-metastatic cutaneous mast cell tumors anywhere on the body and subcutaneous mast cell tumors located at or below the hock or elbow. Treatment of subcutaneous MCT above the hock and elbow may lead to the necrotic material being trapped and unable to create an exit point.
As STELFONTA’s mode of action is different to anything else currently available, it requires a paradigm shift in veterinary treatment from the treating clinician. To understand the biggest change for them, we spoke to a number of clinicians who now regularly offer STELFONTA as a treatment option and we have, summarized their comments below:
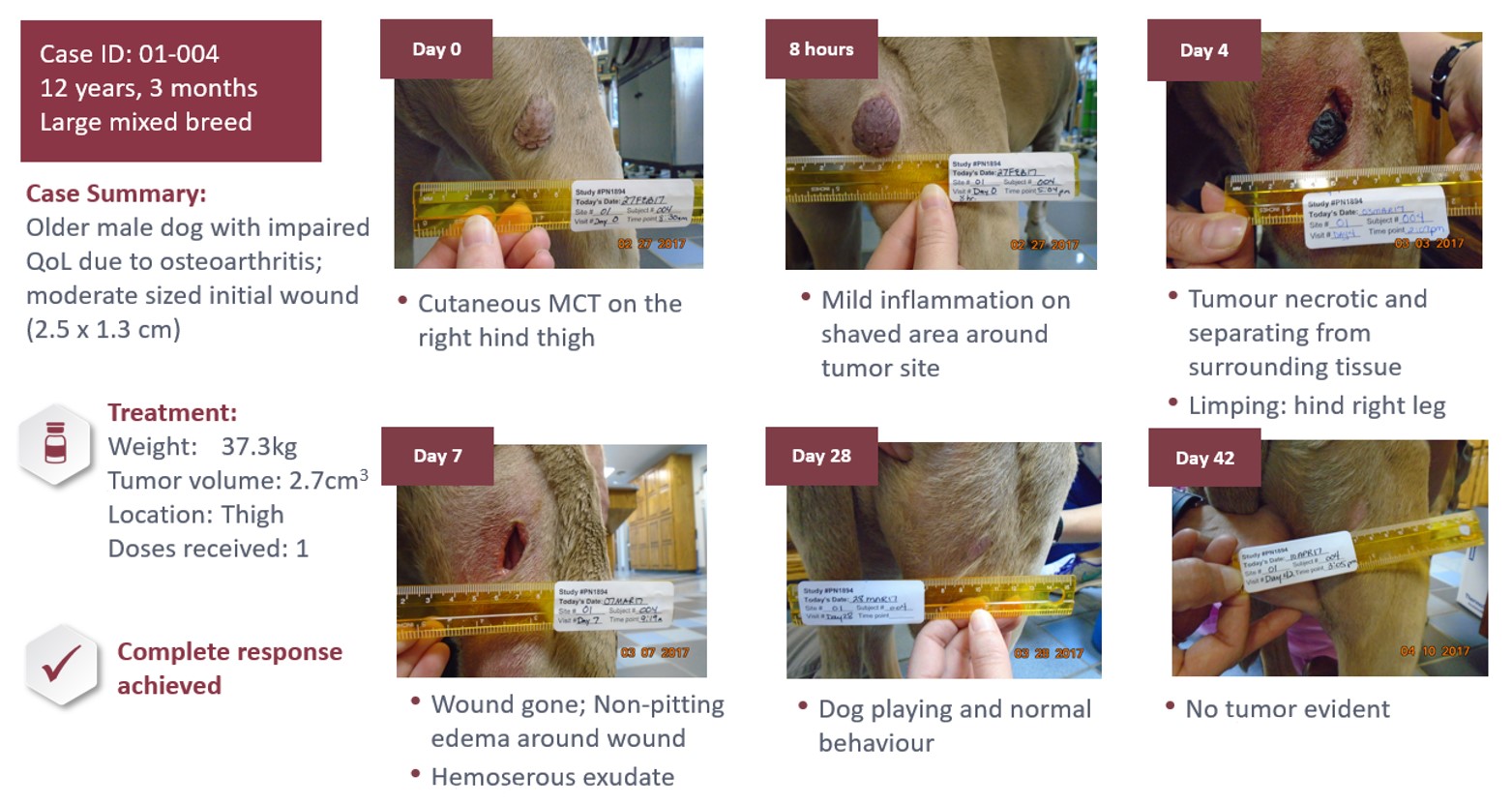
The tumor destruction, wound and healing process are unique to STELFONTA and while individual responses vary, the process typically starts within hours, with many sites (57%) fully healed by day 28 as seen in this case:
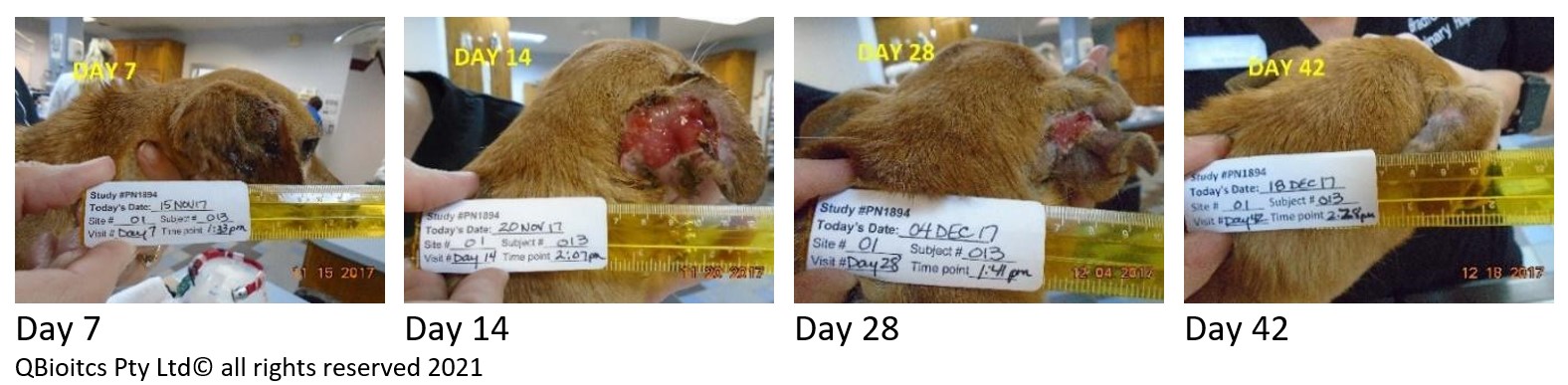
The time taken for the tumor site to heal varies and is correlated to the size of the tumor. While the wound is typically the largest point at day 7, for the below patient the largest wound was noted at day 14.
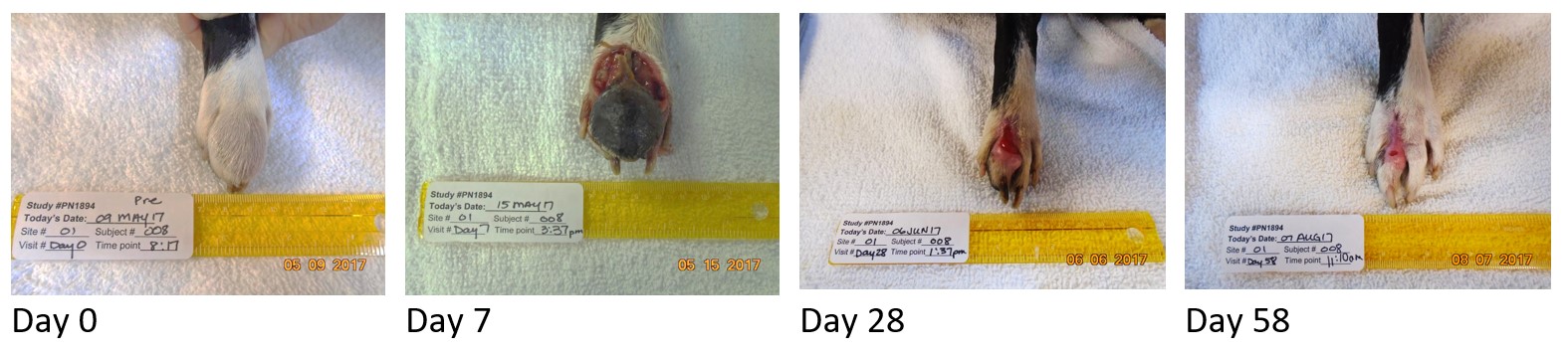
STELFONTA achieves a complete response in 75% of tumors with a single injection. If a complete response has not been achieved by day 28, a second STELFONTA injection may be given, achieving 87% response after one or two treatments. The following patient required a follow up treatment at day 28. The tumor had already reduced in size, and complete response was achieved by day 84.
Full indication and prescribing details are available at https://vet-us.virbac.com/stelfonta.
Talk to your sales representative or click here to order STELFONTA®.